Heads of Health urged people to know the symptoms of allane, as more people have suffered reactions following cosmetic processes related to Botulinum toxin, also known as Botox.
New cases in eastern England and East Midlands indicate the use of an unauthorized Botox -like product, the UKHSA health organization said.
The reactions included severe eyelashes, double vision, difficulty swallowing, speech and lethargy, which can happen weeks after the procedure.
They are not considered to be linked to the cases mentioned in northeast England last month.
There have been 38 cases of medical meal – a rare form of the disease that occurs as a result of medical treatment – reported between June 4 and July 14, according to UKHSA data.
Dr. Gauri Godboole, a medical microbiologist adviser at UKHSA, said: “The variability associated with aesthetic processes is rare, but it may be serious.
“Caused by toxins produced by the Botulinum of the Clostridium bacterium.
“These toxins, but not bacteria, are the active ingredient in botox and similar products.
“Symptoms of sausage can take up to four weeks to grow and if you had a recent Botulinum (botox-like) treatment and have symptoms such as difficulty swallowing or breathing, contact NHS 111 for further advice and seek treatment.”
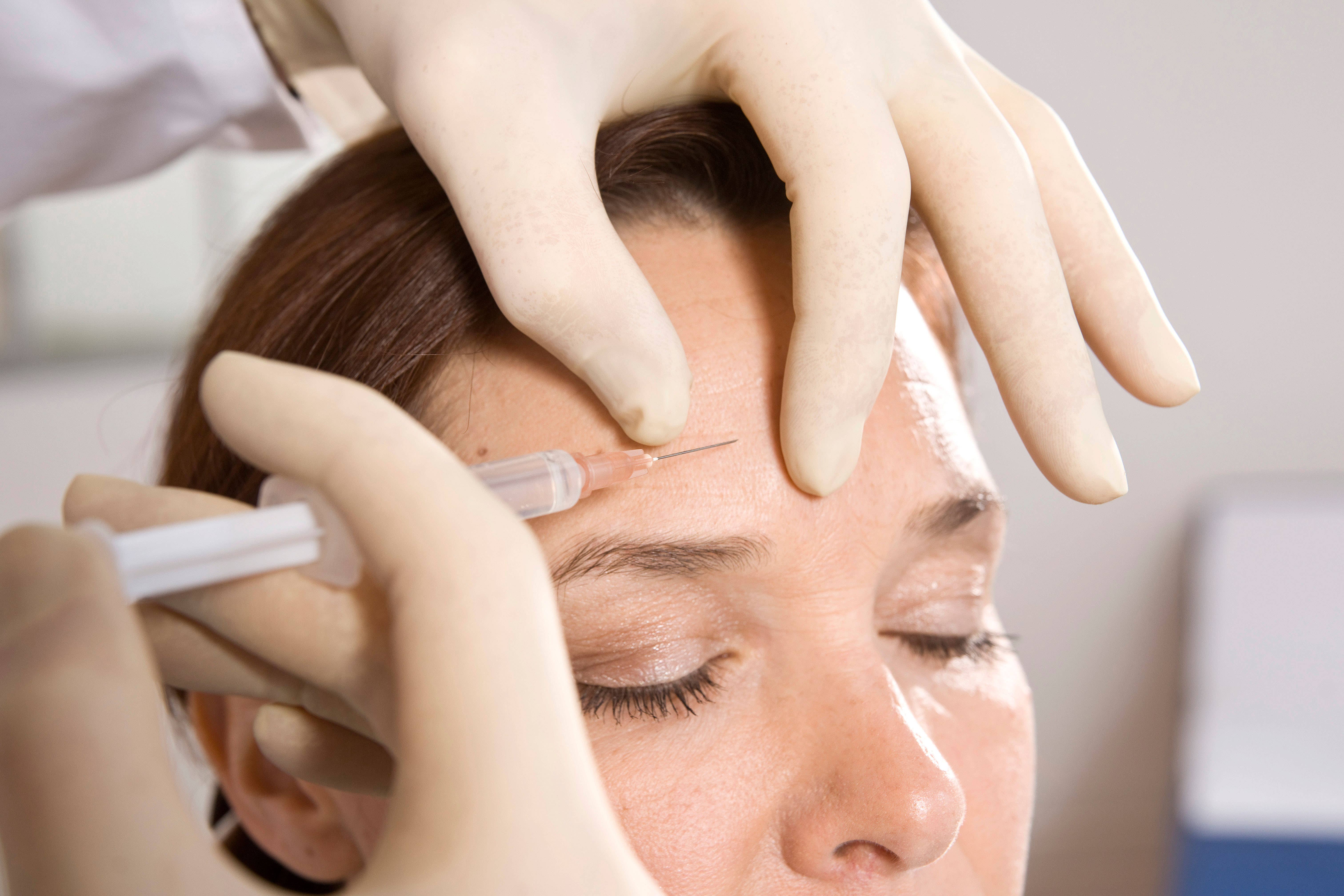
Dr. Godboole also urged someone who is thinking of having cosmetic procedures to check that his professional is licensed.
Professor Meghana Pandit, co-national medical director of secondary care at NHS England, added: “When these procedures go wrong, there is a risk of serious infections and permanent scars, so only registered professionals such as a doctor, nurse or prescriber.
Dr. Alison Cave, CEO of the Registrar of the Drugs and Health Regulatory Organization, warned that “Botulinum is only medical for prescription and should be sold or provided only in accordance with a prescription given by a suitable practitioner, such as a doctor or other specialist.”
He said: “The Botulinum toxin market in any other circumstances significantly increases the risk of getting a product that is either falsified or does not have a license in the UK.
“This means that there are no safeguards to ensure that the products meet MHRA’s standards for quality and safety. Therefore, they can endanger the health of the people who take them.
“Our criminal enforcement unit works hard to identify those involved in illegal drug trade and take strong enforcement action, where necessary, this may include criminal prosecution.”
Practitioners involved in the latter cases have stopped the process and are working with research, UKHSA said.
The organization advises healthcare staff to ensure that it is watching for algae in people who may have had recent aesthetic procedures.
Treatment for symptoms includes anti-toxin.

