.
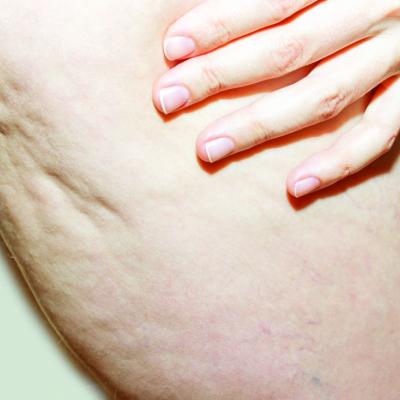
The findings are based on the results of a 12-week study of the device, marketed as Resonic. In that trial of 56 women with moderate to severe cellulite, a single treatment provided a reduction of about 1.01 points on the five-point Cellulitis Severity Scale (CSS) at 12 weeks, which corresponds to about a 29.5% reduction in cellulite from baseline.
The device, which is indicated for long-term improvement in the appearance of cellulite, emits rapid acoustic pulses and shock waves at 50 Hz that are transmitted through the skin. The device “induces the natural shearing of fibrous septa through rapid acoustic pulses,” researchers led by Elizabeth TanziMD, who practices cosmetic dermatology in Chevy Chase, Md., wrote in the follow-up study, which was published in Dermatological Surgery in February “Unlike current treatment options, the device does not require anesthesia or downtime and was well tolerated based on a mean pain score of 2.4 (on a scale of 0–10) during treatment” in the 12-week study, they noted.
To evaluate the long-term effectiveness of the acoustic subtomy device, Dr. Tanzi and her colleagues at four centers prospectively followed 42 patients who participated in the 12-week trial. The study included four visits: screening, one treatment visit, and one follow-up visit 12 weeks after treatment and another after 52 weeks. Because of lockdowns and other reasons related to the COVID-19 pandemic, several participants were unable to make it to follow-up visits and had follow-up visits beyond the 52-week time point, the authors explained.
Blinded panel-certified dermatologists assessed efficacy by correctly identifying post-treatment photographs from the 52-week visit and using a simplified 6-point CSS. They also assessed safety and collected data on participant satisfaction. The mean age of the women was 45.5 years and their mean BMI was 23.9 kg/m2. Blinded reviewers correctly identified the post-treatment photographs at the 52-week visit 95.2% of the time.
Additionally, 70.4% of study participants had at least a 1-point change in CSS score from baseline. Overall, their mean reduction in CSS score from baseline was 1.09 at the 52-week visit and a 34.1% mean reduction in cellulite at that visit, the authors reported.
In other findings, 41 of the 42 study participants (97.6%) rated the improvement in cellulite as good, and 33 (78.6%) agreed that the treatment was relatively pain-free. Immediately after treatment, 85.7% reported an expected adverse event attributable to the device or treatment, which included mild to moderate erythema (76.7%), mild bruising/bruising (5.3%), mild pain (1.7%) and mild heat (1.7%) ). All adverse events resolved without intervention.
The study authors acknowledged some limitations of the study, including the lack of a control group and the inability to differentiate the effectiveness of treatment in the buttocks versus thighs.
A look at devices designed to treat cellulite
“Cellulite is a common complaint among those presenting to cosmetic dermatology clinics, and previous treatment options have been somewhat disappointing in terms of invasiveness, side effects or lack of improvement,” he said. Patricia M. Richey, MDdirector of Mohs surgery at Boston Medical Center, who also conducts research for the Wellman Center for Photomedicine and the Dermatology Laser and Cosmetic Center at Massachusetts General Hospital, Boston.
Acoustic subsurgery “would potentially be a very attractive and unparalleled option given the tolerability and continued clinical improvement after a single treatment,” he told this news outlet. “I agree with the authors that a potential limitation is the lack of comparison between the response in different body regions,” namely the buttocks versus the thighs, he said. “This information would be useful in setting patient expectations, and I suspect that future studies will address this.”
Also asked to comment on the study, Pooja Sodha, MDdirector of the Center for Laser and Aesthetic Dermatology at George Washington University in Washington, D.C., said in an interview that while the results were modest after a single treatment, “there is room for further experimentation to see how the settings, the treatment numbers, the treatment Spacing and site-specific treatment regimens based on tissue depth and tissue zone size/dimple size may improve outcomes.”
He added that the cost of treatment and the correlation with clinical improvement “will become a more real issue when we are going to bring this more widely into the clinics.”
Soliton funded the trial before it was acquired by AbbVie. Dr. Tanzi reported that he had no relevant financial disclosures. Four co-authors reported being employees, consultants or advisory board members or holding stock options in AbbVie. Dr. Richey and Dr. Sodha were not involved in the study and reported having no disclosures.