In the first of a two-part series, Derek Hampton introduces the review by Shrivastava and colleagues (2021), delving into the management of dental biofilm and begins to explore a new protocol – guided biofilm therapy.
Dental biofilm is a polymicrobial entity residing on biotic and abiotic surfaces of the oral cavity (Sanz et al, 2017). These include hard and soft tissues of the oral cavity. Also, surfaces such as orthodontic bands, clear aligners or prostheses (Meto et al, 2019; Lasserre et al, 2018).
Supra and subgingival plaque biofilms can form on the surface of the tooth or implant. Being close to the gingival epithelium, it can negatively affect periodontal and peri-implant health (Lasserre et al, 2018).
Plaque biofilms also form in certain areas of the oral cavity from which it is difficult to remove, thus compromising the oral hygiene management of home care.
Scaling and root planing (SRP) is considered the gold standard for mechanical plaque cleaning (Lindhe et al, 1984). However, it also has disadvantages (Sultan et al, 2017; Rabbani et al, 1981; Eaton et al, 1985).
Today, an alternative new approach is being used to remove biofilm by visualizing it with a disclosing agent and then removing it with a specialized aeroabrasive powder. Then follows the removal of supra and subgingival calculus with the use of specialized instruments. This concept has been called biofilm-guided therapy (Mensi et al, 2020).
Dental biofilm and gum disease
The oral cavity is inhabited by many microbial species, ranging from healthy microorganisms to those with pathogenic potential.
The association between dental plaque and periodontal disease is a well-documented fact.
However, until 1980, it was believed that the microorganism present in dental plaque remained in suspended or planktonic states (Seneviratne et al, 2011).
According to this, the majority of treatment was directed towards the removal of dental plaque. A little later, research showed that microorganisms are not free-floating entities. Rather, they adhere to tooth surfaces (Seneviratne et al, 2011).
It is now widely accepted that microorganisms live in a complex environment known as a biofilm (Seneviratne et al, 2011; Marsh, Zaura, 2017). It is known to be a causative factor in the development of caries and periodontal disease (Marsh and Zaura, 2017; Nimbulkar et al, 2020).
A mature biofilm is a polymicrobial entity composed primarily of bacteria. Although, it can also harbor protozoa, viruses and fungi (Larsen and Fiehn, 2017).
In 2002, Donlan and Casterton defined a biofilm as a non-committal microbial community characterized by cells attached to a substrate, at an interface, or to each other, embedded in an extracellular matrix of polymeric substance that produces and exhibits an altered phenotype, in terms of rate development and gene transcription (Rode et al, 2012).
Biofilm microorganisms exhibit characteristics as a whole unit rather than as individual entities (Berger et al, 2014).
Dysbiosis
Usually, the bacteria found in the biofilm are considered beneficial. However, during the reduced host response predisposed by certain clinical conditions, there is a change in the composition of the microbial flora. Pathogenic bacterial species dominate the healthy microbial flora.
This phenomenon is known as “dysbiosis” (Lasserre et al, 2018).
Biofilm-dwelling bacteria are responsible for the inflammatory cascade and subsequent destruction of supporting tissues (Shrivastava et al, 2021; Shrivastava et al, 2020).
Currently, periodontal and peri-implant diseases are considered to be based on “polymicrobial synergy and dysbiosis” (Hajishengallis and Lamont, 2012).
This has been documented based on the hypothesis that key pathogens such as P. gingivalis are initially introduced into the biofilm.
Later, by undermining the host’s immunity, they manage to modify the composition of the microbial community. This makes it more pathogenic and capable of causing disease (Hajishengallis et al, 2012).
Microbiota
These microbial changes are reinforced by local environmental changes, creating a microbiome capable of sustaining dysbiosis and disease progression.
It is also suggested that rather than directly causing the disease, the underlying pathogens bring about a change in the metabolic activity of the shared traits, which, in turn, increases the pathogenicity of the bacteria and manifests itself in the form of periodontal or peri-implant disease (Darveau, 2010). .
Dysbiosis leads to an increase in the production of inflammatory mediators, which activates the host cell to produce toxic products.
When these toxic products exceed a threshold level, it leads to tissue destruction around the tooth or implant (Lasserre et al, 2018).
In addition, pathogenic bacteria trigger the innate immune response, which attempts to clear the invading microorganism (Silva et al, 2015).
In the innate immune system, pathogens activate pattern recognition receptors (PRRs) that bind to pathogen-associated molecular patterns (PAMPs). These types of receptors include toll-like receptors, nucleotide-binding oligomerization domain (NOD) proteins, group of differentiation 14 (CD14), complement receptor-3, lectins and scavenger receptors (Lasserre et al, 2018; Amano, 2010).
Protein pathways
Toll-like receptors play a critical role in the progression of periodontal/peri-implant inflammation and bone resorption (Kajiva and Kurihara, 2021).
PAMPs have been reported to activate the immune response of T and B cells, leading to the activation of cytokines and an osteolytic pathway (Kajiva and Kurihara, 2021).
In conjunction with innate immunity, the periodontal and peri-implant tissue produces various cytokines and chemokines, which maintain the balance. However, in the presence of dysbiosis, certain cytokines – such as IL-1 – are presentbtumor necrosis factor (TNF)-a and IL-6 – leading to tissue destruction (Lasserre et al, 2018).
In addition to these mechanisms, there are three protein pathways, namely nuclear factor kappa B (NF-KB), cyclooxygenase (COX) and lipoxygenase (LOX), which has an established role in the progression of periodontal and/or peri-implant diseases (Lasserre et al, 2018).
Therefore, understanding its structure and biology is fundamental to discovering the cause and development of periodontal and peri-implant diseases.
For example, biofilm formed on natural tooth or dental implant share a common pattern of microbial colonization (Dihr, 2013).
Biofilm formation is an inevitable phenomenon. However, its control and elimination should not be overlooked, as it is one of the main causes of periodontal and peri-implant diseases.
The need for effective hygiene measures
The dental biofilm is located close to the oral epithelium of the gums. If oral hygiene measures prove ineffective, this supragingival biofilm will accumulate along the gingival epithelium. It could become a potential source of gingival inflammation (Lasserre, 2018; Shrivastava et al, 2021).
Dental biofilm is generally considered to be harmful in nature. If left undisturbed, it can progress to periodontitis, provided there is a concomitant reduced host response (Sahni et al, 2016; Fatima et al, 2021).
To maintain periodontal stability after non-surgical or surgical periodontitis, supportive periodontal therapy (SPT) plays an important role (Ng, 2018).
It is commonly observed that periodontal pockets can easily become colonized with bacteria. This means that regular recall visits in the form of maintenance periodontal treatment are of utmost importance (Renvert and Persson, 2004).
Furthermore, the biofilm formed on the dental implant has a similar microflora to the adjacent tooth (Cortés-Acha et al, 2017).
Periodontal disease
It has been observed that the subgingival microbiota shares common periodontal pathogens as in periodontal disease. Therefore, preservation of the implant by removal of the biofilm should be the primary treatment to combat the development of perimucositis or peri-implantitis.
Oral hygiene is maintained at home through personal care. This includes using a toothbrush with toothpaste (Sahni et al, 2016; Meto et al, 2020). However, despite thorough cleaning, some amount of dental biofilm can be left behind in undetected areas.
Dental anatomical structures – such as the hairpin, cervical enamel projection, deep grooves and pits – can provide a potential biological niche for bacteria (Park et al, 2018).
Professional dental biofilm management will allow professionals to reach hard-to-reach areas where dental plaque remains hidden.
SRP is a gold standard in non-surgical mechanical debridement, based on mechanical biofilm disruption (Lasserre et al, 2018).
Although it is a conventional treatment option, it has its own disadvantages such as being a time-consuming procedure, technically demanding and occasionally uncomfortable for patients (Fleischer et al, 2015).
SRP
In addition, after SRP, the lingual tooth surface and tricuspid areas have been reported to be prone to residual calculus (Rabbani et al, 1981; Eaton et al, 1985).
In addition, furcome regions have been shown to have incomplete root planing (Eaton et al, 1985; Fischer et al, 1991).
Gingival recession and irreversible root damage have also been reported if SRP is performed repeatedly. This is a protocol for supportive periodontal therapy (Sultan et al, 2017).
These disturbing consequences can lead to dental hypersensitivity (Greenstein, 1992).
In addition, it has been observed that the outcome of SRP also depends on the skill level of the clinician (Boyd et al, 2016).
Considering these disadvantages, various technologies have been introduced to remove dental biofilm, such as air polishing.
Introducing guided biofilm therapy
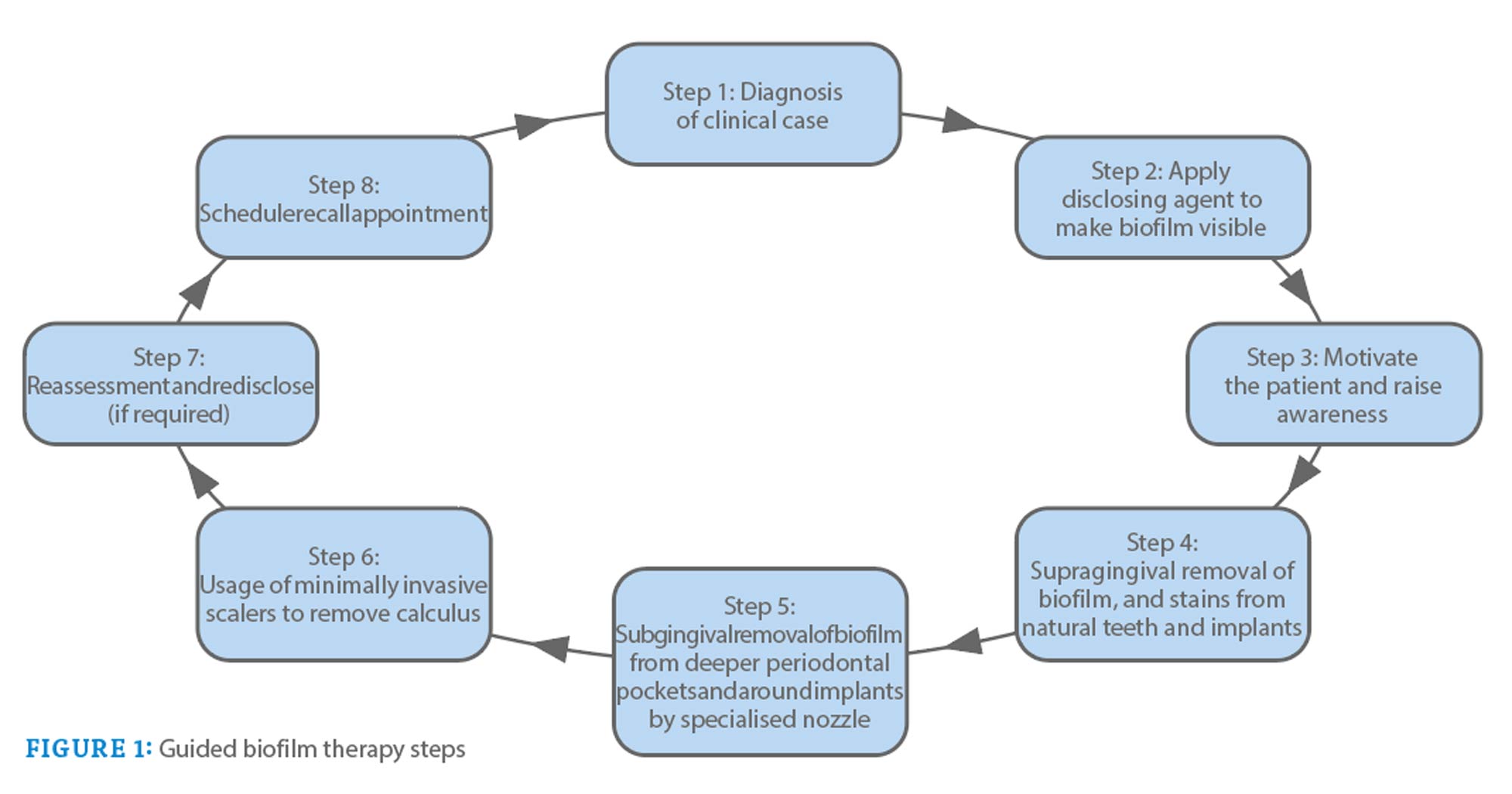
Guided biofilm therapy (GBT) is a new regimen that follows a sequential protocol for plaque and calculus removal. It begins with the detection of biofilms using an unmasking agent. This is followed by the use of aerosanding powder to remove plaque and stains.
The subgingival plaque and calculus are then removed with a specialized nozzle. If required, eventual exfoliation is performed with a specialized tip.
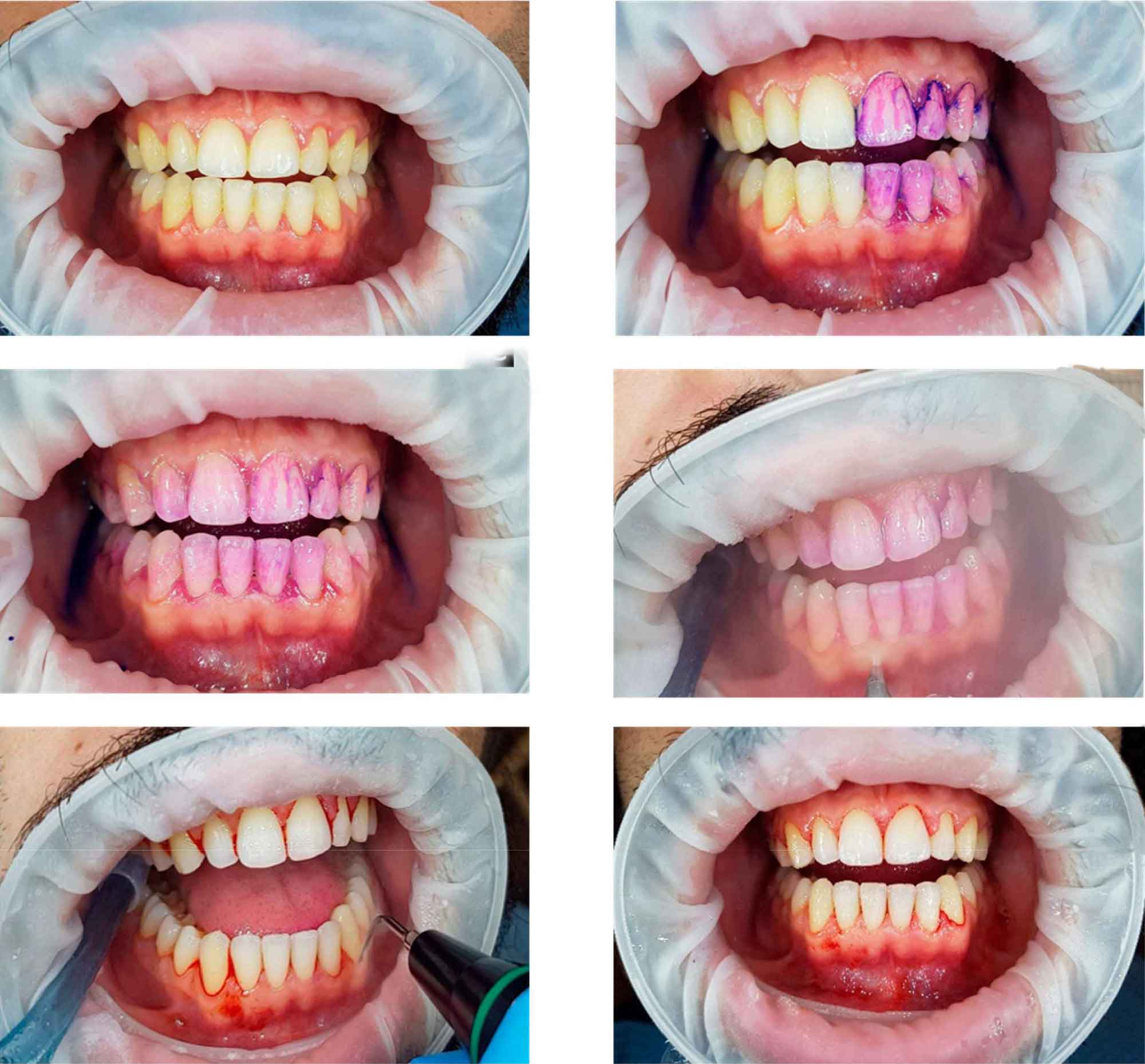
The sequential steps of GBT are described in Figure 1. Figure 2 illustrates the procedure performed in a patient with generalized bleeding on probing, plaque accumulation, and localized stone.
This review will be continued in the next issue. Particular emphasis will be placed on the GBT process and evidence base.
This article appeared on Clinical Dentistry. You can subscribe to the magazine here.